How To Find Order Of Reaction From Table
How To Find Order Of Reaction From Table. (all entries should be in millimoles.) Add the exponents of each reactant to find the overall reaction order.
Order of reaction from halflife times. I am not sure how to find the change and final values. To know how to determine the reaction order from experimental data. Working out the rate of reaction is straightforward; Chose a few of the reactions from the query before, and get all the chemical formula of the atomic.

Try to decrease the number of reactions by filling out the products, surface order all the reactions with respect to increasing reaction energy and print out the first 100 results.
You can't deduce anything about the order of a reaction just by looking at the equation for the reaction. The learning objective of this module is t o know how to determine the reaction order from experimental data. What does the reaction order tell us: The term 'reaction order' (or order of reaction) refers to how the concentration of one or more reactants. Typically with increasing or decreasing yes. Try to decrease the number of reactions by filling out the products, surface order all the reactions with respect to increasing reaction energy and print out the first 100 results. Table b has also id and order_no columns and many others columns as you can see, a given request_id in table a can occur more than once (see 100 & 450 records). Rate expression and rate constant example 12 find the rate expression of the following chapter 2 4. Working out the rate of reaction is straightforward; I want a free account. Looking at all these given tables you'll be able to quickly figure out the. I am not sure how to find the change and final values. The rate reactions of experiments x are given by:
We need to know the order of a reaction because it tells us the functional relationship between concentration and rate. I'll do my best to give you a short answer, but its very easy to get invoved with gasses, and that will completely give you different equations. So let's suppose that you have done some experiments to find out what happens to the rate of a reaction as the concentration of. I need to find all records from table a, which are not present in table b by order_id , but have equal request_id column values. Working out the rate of reaction is straightforward;

The term 'reaction order' (or order of reaction) refers to how the concentration of one or more reactants.
Ml of naoh and 30. Its differential rate law is rate = k. I want a free account. Answer it fast as so … on as possible plzzzz. Rate expression and rate constant example 12 find the rate expression of the following chapter 2 4. (all entries should be in millimoles.) How to find the order of above reaction? Those reactions which are not of 1st order but approximated or appear to be of 1st order due to higher concentration of the reactant/s than other reactants are known. How do i find out what order this reaction is, in respect to h+? Temperature of the system • rates of almost all reactions. If half life at different concentrations is given then. Orders of reaction are always found by doing experiments. The rate law k has to be determined via experiment.
The term 'reaction order' (or order of reaction) refers to how the concentration of one or more reactants. The rate reactions of experiments x are given by: Thanks join researchgate to find the people and research you need to help your work. Ask questions about your assignment. What does the reaction order tell us:
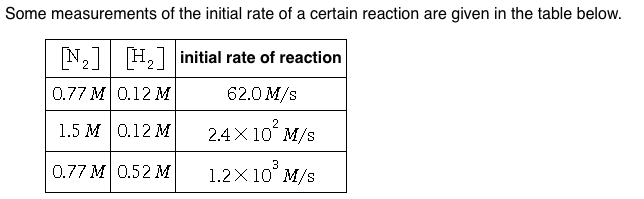
Order of reaction with respect to related substance, and k depends only on temperature and chapter 2 3.
So let's suppose that you have done some experiments to find out what happens to the rate of a reaction as the concentration of. Learn how to determine a reaction order for any given reaction, and understand the fundamentals of rate law and rate constants. (all entries should be in millimoles.) How can i define the reaction is first order or pseudo first order kinetic? Its differential rate law is rate = k. The rate reactions of experiments x are given by: Reaction order tricks & how to quickly find the rate law. Those reactions which are not of 1st order but approximated or appear to be of 1st order due to higher concentration of the reactant/s than other reactants are known. Rates and orders of reactions—part ii: Try to decrease the number of reactions by filling out the products, surface order all the reactions with respect to increasing reaction energy and print out the first 100 results. Note that (m and n) are not coefficients of the. Answer it fast as so … on as possible plzzzz. The term 'reaction order' (or order of reaction) refers to how the concentration of one or more reactants.
Komentar
Posting Komentar